In simple terms, acid rain formation reaction as follows:
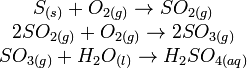
Evidence of an increase in acid rain derived from the analysis of polar ice. Looks pH levels to drop since the start of the Industrial Revolution from 6 to 4.5 or 4. Other information obtained from organisms known as diatoms which inhabit ponds. After many years, the dead organisms will settle in layers of sediment in the bottom of the pool. Diatom growth will increase at a certain pH, so the number of diatoms found in the bottom of the pool will show the pH changes on an annual basis when we look into each of these layers.
Since the start of the Industrial Revolution, the amount of emissions of sulfur dioxide and nitrogen oxides into the atmosphere is increasing. Industries that use fossil fuels, especially coal, a major source of sulfur oxides is increasing. PH readings in industrial areas are sometimes recorded up to 2.4 (the acidity of vinegar). These sources, plus the transportation, are major contributors to acid rain.
The problem of acid rain not only increased in line with population growth and industrial but has evolved to become more widespread. The use of a high chimney to reduce local pollution contribute to the spread of acid rain, due to the release of greenhouse gas emissions will go to the regional air circulation which have greater reach. Often, acid rain occurs in areas far from the source, where the mountainous regions tend to earn more because of high rainfall here.
There is a close relationship between low pH with decreasing fish populations in lakes. pH below 4.5 is not possible for fish to live, while pH 6 or higher will help the growth of the fish population. Acid in the water will inhibit the enzyme production of trout larvae to come out of their eggs. Acid also bind toxic metals in the lake are like aluminum. Aluminum will cause some fish secrete excessive mucus around the gills so that the fish could hardly breathe. Phytoplankton growth is the source of fish food is also inhibited by high pH levels.
Plants affected by acid rain in various ways. Waxy coating on the leaves is broken so that the nutrients disappear so the plants are not resistant to the cold, fungi and insects. Root growth slows so fewer nutrients that can be taken, and essential minerals to be lost.
The ions are separated due to toxic acid rain became a major threat to humans. Copper in water affects outbreaks of diarrhea in children and aluminum contaminated water can cause Alzheimer's disease.
0 komentar:
Posting Komentar