Because of the chemical reactions within the cells, the capacity of a battery depends on the discharge conditions such as the magnitude of the current (which may vary with time), the allowable terminal voltage of the battery, temperature, and other factors. The available capacity of a battery depends upon the rate at which it is discharged. If a battery is discharged at a relatively high rate, the available capacity will be lower than expected.
The capacity printed on a battery is usually the product of 20 hours multiplied by the constant current that a new battery can supply for 20 hours at 68 F° (20 C°), down to a specified terminal voltage per cell. A battery rated at 100 A·h will deliver 5 A over a 20-hour period at room temperature. However, if discharged at 50 A, it will have a lower capacity.
The relationship between current, discharge time, and capacity for a lead acid battery is approximated (over a certain range of current values) by Peukert's law:
 is the capacity when discharged at a rate of 1 amp.
is the capacity when discharged at a rate of 1 amp. is the current drawn from battery (A).
is the current drawn from battery (A).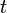 is the amount of time (in hours) that a battery can sustain.
is the amount of time (in hours) that a battery can sustain.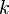 is a constant around 1.3.
is a constant around 1.3.
Internal energy losses and limited rate of diffusion of ions through the electrolyte cause the efficiency of a real battery to vary at different discharge rates. When discharging at low rate, the battery's energy is delivered more efficiently than at higher discharge rates, but if the rate is very low, it will partly self-discharge during the long time of operation, again lowering its efficiency.
Installing batteries with different A·h ratings will not affect the operation of a device (except for the time it will work for) rated for a specific voltage unless the load limits of the battery are exceeded. High-drain loads such as digital cameras can result in delivery of less total energy, as happens with alkaline batteries. For example, a battery rated at 2000 mAh for a 10- or 20-hour discharge would not sustain a current of 1 A for a full two hours as its stated capacity implies.

0 komentar:
Posting Komentar